Submitting Applications - Step by Step
Application Forms
Please use our application forms to request the use of data and/or biospecimen from the DZHK Heart Bank:
- Subform A (liquid biospecimen, imaging data)as of 04/2023
- Subform B (DNA, RNA)as of 04/2023
- Subform C (tissue) as of 12/2022
- Specification Participant Collective(s)as of 09/2023
- Specification data for project analysisas of 09/2023
Regular video consultation
Tuesdays in 2-week rhythm, 1:00-2:00 p.m.
3.10. cancelled (public holiday) | 17.10. | 31.10. | 14.11. | 28.11. | 12.12.
Registration: use.access(at)dzhk.de
Link to Zoom Meeting
DZHK Use and Access Policy
The DZHK Use and Access Policy regulate that data and biospecimen beyond the study question can be collected and made available for further research. This makes it an elementary component of the DZHK's added value in the funding of clinical studies.
Contact:
DZHK Use and Access Office
Alexandra Klatt
Tel: 030 3465 529-10
use.access(at)dzhk.de
DZHK Transfer Office
Sabine Hanß, Tabea Scharfe
use.access(at)dzhk.de
DZHK Heart Bank – The Cardiovascular Research Resource
To create and submit applications for use of data (clinical data, imaging data, Omics data) and/or biospecimens, please proceed as follows:
What you need to consider before submitting an application
Step 1 - Check out our Use and Access Policy.
Step 2 - Get an overview of the different resources available and daa (clinical data, imaging data, OMICs data) and/or biospecimen they contain. Select the appropriate resource(s) to answer your research question.
How to Apply
Step 3 - Fill in the application form.
Step 4 - Fill in the subform(s). The subforms contain specifications for the respective DZHK Heart Bank resource.
Step 5 - Fill in the specification lists if you want to use the resource with liquid specimen and image data. You can get an overview of available data in the Feasibility Explorer and a detailed description with the Data Catalogue.
Step 6 - Combine all forms into one application and submit everything to the Use and Access Office by e-mail.
Submission Deadlines
Requests for use can be submitted on a continuous basis. Review always takes place on the 15th of the month. Only complete applications can enter the further Use and Access process.
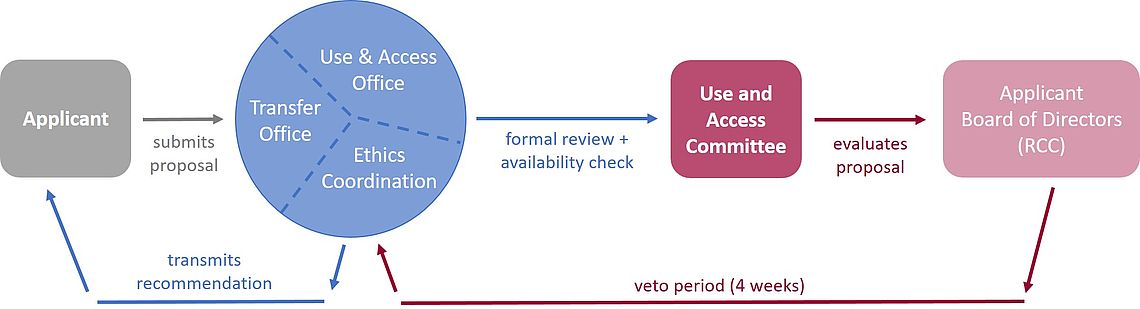
What Happens After Submission
After submission, usage requests are first checked for formal criteria by the Use and Access Office and then passed on to the DZHK transfer office for the collective check of available data and biosamples. This availability statement is then evaluated together with the respective application for use by the Use and Access Committee and a recommendation is made.
What to consider for release and shipment of biospecimens
Please follow the instructions for the release and shipping process of biospecimens as described in DZHK-SOP-P-03 Release and Shipping of Biospecimens for Secondary Use Projects; valid for DZHK Clinical Study Units.
Download DZHK-SOP-P-03 Release of biospecimens for secondary use projects V1.2

