The determination of the maximum safe dose could be completed after the succesful treatment of 10 patients. A total of 35 patients will now be treated with artificial heart tissue consisting of 800 million heart cells in order to gain insights into the efficacy of the heart patch. The BioVAT-HF-DZHK20 study is being conducted at the University Medical Center Göttingen (UMG) and the University Medical Center Schleswig-Holstein (UKSH), Campus Lübeck, and is funded by the German Centre for Cardiovascular Research (DZHK) as well as Repairon GmbH.
The BioVAT-HF-DZHK20 clinical trial will begin in early 2021 and will be the first human trial to test whether artificial heart tissue can permanently strengthen the heart muscle of patients with severe heart failure by rebuilding heart muscle tissue. The tissue will be produced from induced pluripotent stem cells (iPSCs) at the Göttingen University Medical School under the direction of the Department of Transfusion Medicine and will be sewn onto the diseased heart muscle as a so-called heart patch. The first phase of the dose-finding study focused on testing the feasibility and safety of the method. The aim of the clinical trial is to obtain approval for the use of artificial heart tissue as a medicine for new therapies.
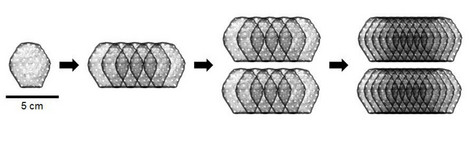
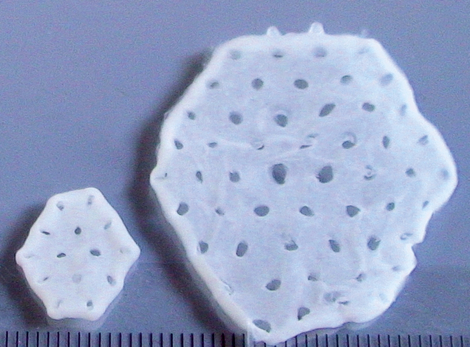
Heart patch made from up to 800 million cells
In order to determine the maximum safe dose, two patients were implanted with a heart patch consisting of 200 million cells, two patients with a heart patch consisting of 400 million cells and six patients with a heart patch consisting of 800 million cells. This was the maximum dose as per the study protocol and completed Phase 1 of the BioVAT-HF study, the dose finding phase. A total of six billion heart cells derived from pluripotent stem cells were implanted in the dose-finding phase.
"The heart patch produced at the University Hospital in Göttingen can be implanted in patients with heart muscle disease. For the first time, we were able to observe the formation of real heart muscle in the human heart. We are now looking forward to the further evaluation of the clinical data after completion of the dosage determination," said Prof. Dr. Wolfram Hubertus Zimmermann, Director of the Institute of Pharmacology and Toxicology at the UMG and scientific head of the BioVAT-HF study.
How well does an 800 million cell patch work?
The next step will be to continue the BioVAT-HF trial as a proof-of-concept (PoC) study. A first interim analysis focusing on efficacy will be performed after the treatment of 15 patients with a dose of 800 million cells. This data is expected in the second half of 2024. A total of 35 patients will be treated in the PoC study.
The BioVAT-HF-DZHK20 study is being conducted at the University of Göttingen under the scientific leadership of Prof. Dr. Wolfram Hubertus Zimmermann, Institute of Pharmacology and Toxicology at the UMG and spokesperson of the DZHK Göttingen site, and under the clinical leadership of Prof. Dr. Tim Seidler, Clinic for Cardiology and Pneumology at the UMG. In addition to clinical partners from the University of Göttingen, the study also includes the Clinic for Heart and Thoracic Vascular Surgery at the University Hospital Schleswig-Holstein (UKSH), Campus Lübeck.
The trial follows 25 years of pre-clinical development. The German Centre for Cardiovascular Research (DZHK) supported the final developmental steps before clinical use as part of its translational strategy. The production of the artificial heart tissue for the use in BioVAT-HF at the UMG is being co-financed by the Göttingen-based biotech company Repairon GmbH.
"We see for the first time the formation of real heart muscle in the human heart"
"We are delighted to be able to announce the completion of the dose finding in the BioVAT-HF study, which is investigating a fundamentally new form of treatment for severe heart muscle weakness," said Prof. Dr. Tim Seidler from the Heart Centre at the University of Göttingen and principal investigator of the clinical trial.
"The transplantation of artificial heart tissue is a new therapeutic option for patients with severe myocardial insufficiency, which may serve in the future as an alternative to mechanical heart support systems," says Prof. Dr. Ingo Kutschka, Director of the Clinic for Heart, Thoracic and Vascular Surgery at the University of Göttingen and surgical head of the BioVAT-HF trial at the UMG.
"After many years of preclinical development and research, we have now been able to demonstrate for the first time ever the transferability of biological myocardial tissue implants and the formation of new musculature in the human heart in patients with severe myocardial insufficiency," says Prof. Dr. Stephan Ensminger, Director of the Clinic for Cardiac and Thoracic Vascular Surgery at the University Heart Centre Lübeck (UHZL) of the University Hospital Schleswig-Holstein (UKSH) and surgical director of the BioVAT-HF trial at the UKSH.
"For the first time, we are seeing the development of real heart muscle tissue in the human heart with severe heart muscle weakness and are eagerly awaiting the results of the BioVAT-HF study," says Prof. Dr. Gerd Hasenfuß, Chairman of the Heart Centre at the University of Göttingen.
"We are delighted to be participating in the world's first human trial of the BioVAT procedure, which aims to address the medical undersupply of patients with severe heart muscle weakness," says Prof. Dr. Ingo Eitel, Director of the Medical Clinic II (Cardiology, Angiology and Intensive Care) at the University Heart Centre Lübeck (UHZL) of the UKSH.
"There is a great need for novel, reparative treatment options for patients with severe heart muscle weakness," said Prof. Dr. Wolfram-Hubertus Zimmermann, Director of the Institute of Pharmacology and Toxicology at the University Medical Center Göttingen (UMG), Co-founder of Repairon GmbH and scientific leader of the BioVAT-HF study. "After more than 25 years of development, the BioVAT-HF trial is the first study to investigate whether the implantation of lab-grown heart muscle represents a new treatment option for patients with untreated heart muscle disease. The novel approach is designed to address the central cause of heart muscle failure, the loss of heart muscle cells. Our observations to date with BioVAT-HF are consistent with the results of extensive pre-clinical studies."
Study: Safety and efficacy of induced pluripotent stem cell-derived engineered human myocardium as biological ventricular assist tissue in end-stage heart failure, BioVAT-DZHK20
Scientific contact: Prof. Dr. Wolfram-Hubertus Zimmermann, University of Göttingen, Georg August University, Institute of Pharmacology and Toxicology, 0551 39-65781, biovat.info(at)med.uni-goettingen.de
Prof. Dr. Stephan Ensminger, University Hospital Schleswig-Holstein, University Heart Centre Lübeck
Clinic for Heart and Thoracic Vascular Surgery, 0451 500-42301, stephan.ensminger(at)uksh.de
Prof Dr Ingo Eitel, University Hospital Schleswig-Holstein, Medical Clinic II, 0451 500-44501, ingo.eitel(at)uksh.de
Press contact: Christine Vollgraf, Press and Public Relations, German Center for Cardiovascular Research (DZHK), Tel: 030 3465 529 02, presse(at)dzhk.de
Further information: www.biovat.dzhk.de