According to the 2022 German Heart Report, heart failure leads to around 400,000 hospital admissions per year in Germany, making it the most common reason for hospitalisation. In ten per cent of all patients with heart failure, the condition is so severe that it is associated with an average life expectancy of just twelve months despite optimised treatment. Due to demographic change, the incidence of heart failure will continue to increase, leading to the death of more people than any other disease. Novel treatment options for the repair or regeneration of the heart would significantly expand the range of therapies.
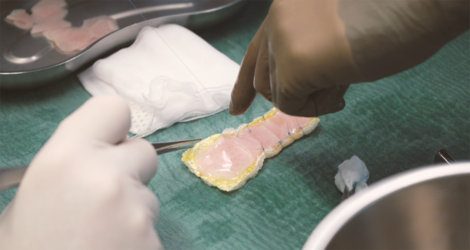
The clinical study BioVAT-HF-DZHK20, jointly conducted by the University Medical Center Göttingen (UMG) and the University Hospital Schleswig-Holstein (UKSH), University Heart Center, Lübeck Campus, has been testing for the first time in humans since the beginning of 2021 whether heart tissue grown in the laboratory can permanently strengthen the heart of patients with severe heart failure by rebuilding heart muscle tissue. The tissue is produced from induced pluripotent stem cells in clean rooms at the UMG under the direction of Transfusion Medicine and sewn onto the diseased heart muscle as a so-called heart patch. The aim of the clinical trial is to obtain authorisation for the use of cultivated heart tissue as a drug for innovative therapies.
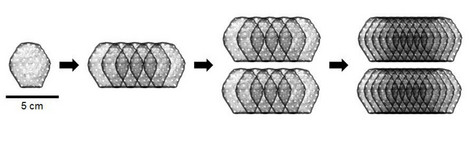
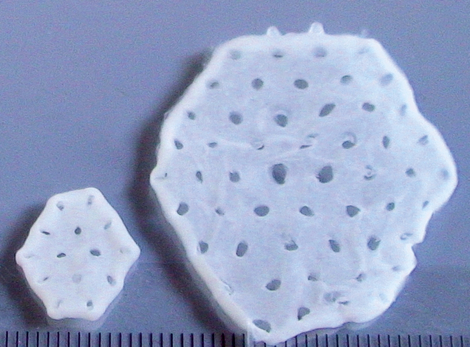
In the first phase of the study, the maximum safe dose for the heart patch was determined: two layers of ten overlapping heart tissues each consisting of a total of 800 million heart cells. A total of eight patients have been implanted with this maximum dose of cultured heart tissue at the UMG and UKSH since April 2022. One of these study patients is Frank Teege, who underwent surgery at the UKSH: "I was getting weaker and weaker and couldn't walk 50 metres without getting short of breath. I was then diagnosed with heart failure. In fact, I only had a cardiac output of ten per cent," says the now 66-year-old. "At the University Heart Center Lübeck of the UKSH, I was also given the opportunity to take part in the heart patch study. It was the right step for me. After the operation with the heart patch, my cardiac output has improved significantly. It is now at 35 per cent."
For the scientific director of the BioVAT-HF-DZHK20 study, Prof Dr Wolfram-Hubertus Zimmermann, Director of the Institute of Pharmacology and Toxicology at the UMG, Frank Teege's story confirms that the heart patches produced at the UMG can be successfully implanted in patients with cardiac insufficiency. "For the first time, we were able to observe the development of real heart muscle in the human heart. I am very pleased that Frank Teege is benefiting from the treatment and that we can see an improvement in his state of health," says Prof Dr Zimmermann. "The study is also an example of translational research in and from Göttingen, i.e. the transfer of findings from the laboratory to clinical application in patients. Our goal is to establish the heart patch as a treatment method for patients with advanced heart failure - worldwide," says Prof Dr Zimmermann.
"After many years of preclinical development and research, we have now been able to demonstrate for the first time the transferability of biological heart muscle tissue implants and the development of new muscle in the human heart with severe heart muscle weakness," says Prof. Dr Stephan Ensminger, Director of the Clinic for Cardiac and Thoracic Vascular Surgery at the University Heart Center Lübeck of the UKSH and surgical director of the BioVAT-HF-DZHK20 study at the UKSH.
"The successful treatment of Frank Teege shows that we are on the right track with the heart patch. Two years after the transplantation of artificial heart tissue, there have been significant improvements in his overall condition. This may provide a new therapeutic option for patients with severe cardiac insufficiency. In the future, a laboratory-grown tissue transplant could become an alternative to mechanical heart support systems for some patients," says Prof Dr Ingo Kutschka, Director of the Department of Cardiothoracic and Vascular Surgery at the UMG and Surgical Director of the BioVAT-HF-DZHK20 study at the UMG.
In the current second phase, the BioVAT-HF-DZHK20 study is being continued as a proof-of-concept (PoC) study. An initial interim evaluation with a particular focus on efficacy will be carried out after treating a total of 15 patients with a dose of 800 million cells. This data is expected in the second half of 2024. A total of 35 patients are to be treated in this way.
Background BioVAT-HF-DZHK20
The BioVAT-HF-DZHK20 clinical trial started at the beginning of 2021 and is part of the translational research programme of the German Center for Cardiovascular Research (DZHK). In the first phase of the study, twelve patients were treated with the heart patch, six each at the UMG and the UKSH. In order to determine the maximum safe dose, two patients were implanted with heart patches made from 200 million cells, two patients with heart patches made from 400 million cells and eight patients with heart patches made from 800 million cells. The highest dose according to the study protocol was thus achieved and phase 1 of the BioVAT-HF-DZHK20 study was completed. A total of 7.6 billion pluripotent stem cell-derived heart cells were implanted during the dose-finding phase.
The study was preceded by 25 years of preclinical development. The production of the artificial heart tissue for use in the BioVAT-HF-DZHK20 study at the UMG is co-financed by the Göttingen-based biotechnology company Repairon GmbH. Repairon GmbH, a spin-off from the University Medical Center Göttingen, intends to apply for approval of the heart patch treatment.
Video about the heart patch (in German)
Scientific contact: Prof Dr Wolfram-Hubertus Zimmermann (biovat.info@med.uni-goettingen.de), Institute of Pharmacology and Toxicology, University Medical Center Göttingen
Source: Press release UMG (in German only)