Facilitating International Clinical Trials
Multinational Clinical Trials Initative:
British Heart Foundation
Dutch Heart Foundation(Hartstichting)
Deutsches Zentrum für Herz-Kreislauf-Forschung
Institute of Circulatory and Respiratory Health, Canada
National Heart Foundation of Australia
National Heart Foundation of New Zealand
National Heart, Lung and Blood Institute, USA
Swiss Heart Foundation

Contact
Prof. Dr. Thomas Eschenhagen
t.eschenhagen(at)uke.de
For administrative and procedural issues: DZHK head office, Clinical Research Coordination Group, clinicalstudies(at)dzhk.de
Supporting multinational clinical trials is a focus of the 2021 established Global Cardiovascular Research Funders Forum (GCRFF). The Initiative is a sub-group of 8 of the 12 national funders in 10 countries.
It aims to facilitate multinational clinical trials by bringing all potential funders to the table right from the start. Scientists simply submit an "Expression of Interest" (EOI) - the initiative coordinates the matching of national funders.
The process has clear advantages for scientists and funders:
- All funding organisations can get involved in the study design from the beginning. Until now, it has usually been the case that other funding organisations are only brought on board once the study has already been started with one funding organisation.
- The study can be coordinated and started simultaneously in different countries: this increases the chances of success.
- Reduce the risk to the funder(s) because there is greater assurance that the trial will be deliverable and costs are spread.
Want to submit an expression of interest (EOI)?
How it Works
Applicants from Germany do not necessarily have to be members of the DZHK.
> Not a DZHK member?
If you are not a member of the DZHK, and are therefore not eligible to apply for funding from the DZHK, you should plan to apply for national funding for the German component of your study, for example from the Federal Ministry of Education and Research (BMBF) or the German Research Foundation (DFG).
The EOI is submitted via the Grants Management System der British Heart Foundation (BHF).
> Member of the DZHK?
DZHK PIs, DZHK scientists, members of the Young DZHK, and scientists who are members of one of the Cardiological Competence Networks associated with the DZHK are eligible to apply for funding from the DZHK. (Note: The funding recipient should be the respective DZHK partner institution, or one of the registered associations of the Cardiological Competence Networks.)
A maximum of 2.5 million euros can be applied to the DZHK (for the German study part). The DZHK can only fund a very limited number of studies (maximum 1-2 positive funding decisions per year).
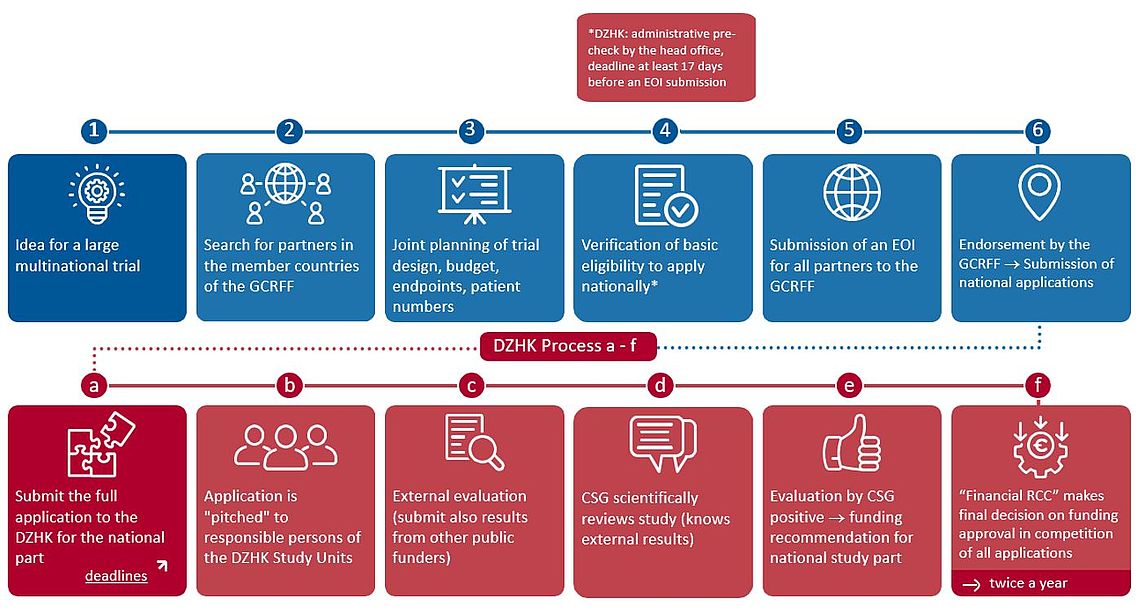
3 Steps to promote the German component of the study by the DZHK
If you are applying to the DZHK for funding for the German component of the study, please proceed as follows:
1. pre-check of the EOI: have the DZHK check your eligibility to apply.
The pre-check takes about 14 days.
EOI for a formal pre-check to clinicalstudies(at)dzhk.de (Clinical Research Group):
- Is the scientist basically established in the DZHK and eligible to apply?
- Does the EOI follow the rules outlined in the DZHK funding scheme for guideline-relevant studies?
- Are there overlaps with a DZHK-funded study?
Note: The formal review is no guarantee for funding by the DZHK. Only if a full proposal (see step 3) receives funding approval from the Research Coordinating Committee (RCC) will the DZHK fund the German part of the study.
2. submit EOI to the GCRFF
The current deadlines can be found at www.bhf.org.uk
If the formal preliminary check is positive, the EOI including the formally confirmed eligibility can be submitted to the GCRFF. If the check is not positive, another German funding body must be approached for the German part of the study.
The EOI is submitted via the Grants Management System of the British Heart Foundation (BHF).
3. submit a full proposal to the Clinical Study Group (CSG) of the DZHK.
See chart "DZHK process a-f".
Here you will find the next deadline, information and application documents (DZHK intranet, login required) for a full proposal.
The study will be presented to the DZHK clinicians in a short clinical "pitch" to test the feasibility in DZHK Study Units.
Once the GCRFF has endorsed the study, a full proposal can be submitted to the DZHK Clinicial Study Group (CSG). The CSG reviews the full proposal in the regular procedure.
Note: An endorsement by the GCRFF is no guarantee for funding by the DZHK - but it improves the chances that the German part of the study will be funded by the DZHK.
What must be included in the EOI?
> Please note, the EOI must include co-applicants from the countries of the national funders who have been named in the funding request. Co-applicants should have been fully engaged in the development of the study and should have inputted into the EOI.
> If you have already secured funding for elements of the study, please provide details (funders name, amount awarded, date of award) in the EOI.
> Also, outline any formal applications for funding that have been submitted, where a result is awaited. Give the details of the funder, funding scheme and the date when a decision will be reached.
Detailed information on the process and criteria can be found on the British Heart Foundation website:
How it works
Criteria for submitting an Expression of Interest
How to apply
Deadlines & Panel Dates 2024
26th JAN 2024, 10:00 (CET): Submit EOI
21st MAR 2024: Expert Advisory Panel meeting
24th MAY 2024, 10:00 (CET): Submit EOI
18th JUL 2024: Expert Advisory Panel meeting
27th SEP 2024, 10:00 (CET): Submit EOI
26th NOV 2024: Expert Advisory Panel meeting
Deadlines & Panel Dates 2025
24th JAN 2025, 10:00 (CET): Submit EOI
20th MAR 2025: Expert Advisory Panel meeting
23rd MAY 2025, 10:00 (CET): Submit EOI
17th JUL 2025: Expert Advisory Panel meeting
26th SEP 2025, 10:00 (CET): Submit EOI
25th NOV 2025: Expert Advisory Panel meeting
The EOI is submitted via the Grants Management System of the British Heart Foundation (BHF):
Please Note
The GCRFF Multinational Clinical Trials Initiative provides a mechanism to support better coordination of consideration of multinational trial funding. It does not fund trials directly, nor does it guarantee funding by individual funders.
About the Global Cardiovascular Research Funders Forum (GCRFF)
We've joined forces with leading cardiovascular research funders in a new global partnership. The Global Cardiovascular Research Funders Forum (GCRFF) aims to accelerate the pace of progress by creating opportunities for cross-border coordination and collaboration between world-leading cardiovascular researchers and organisations. Its members will initially collaborate by sharing information on research funding priorities, strategic initiatives, and clinical trials.
The Forum includes many of the biggest independent funders of cardiovascular research in the world, which together support more than US$600 million in research annually.
The 12 members in 10 countries are:
Australia - National Heart Foundation of Australia
Canada - The Heart and Stroke Foundation of Canada; Institute of Circulatory and Respiratory Health, Canadian Institutes of Health Research, Canada
Denmark - Danish Heart Foundation (Hjerteforeningen)
France - Leducq Foundation
Germany - German Centre for Cardiovascular Research
Netherlands - Dutch Heart Foundation (Hartstichting)
New Zealand - National Heart Foundation of New Zealand
Switzerland - Swiss Heart Foundation
UK - British Heart Foundation
USA - American Heart Association; National Heart, Lung and Blood Institute
Despite the astonishing progress that cardiovascular research has made in recent decades, cardiovascular diseases remain the biggest cause of death and disability globally, meaning urgent progress is needed.
International funders aim to improve global heart health (22.06.2021) press release