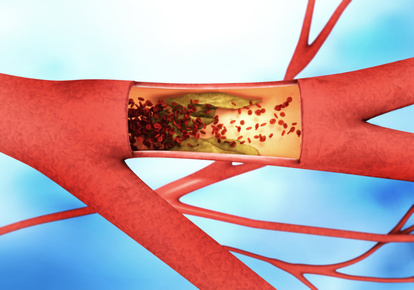
Atherosclerosis occurs when cholesterol deposits build up in the walls of blood vessels and inflammatory cells invade the vessel wall. This leads to a damaging inflammatory reaction that becomes self-sustaining and chronic, resulting in deposits known as plaques. They constrict the blood vessels. And even more: Blood clots can form on the plaques, break off, travel through the vasculature and block the blood vessels in the heart or brain. This can lead to a heart attack or stroke.
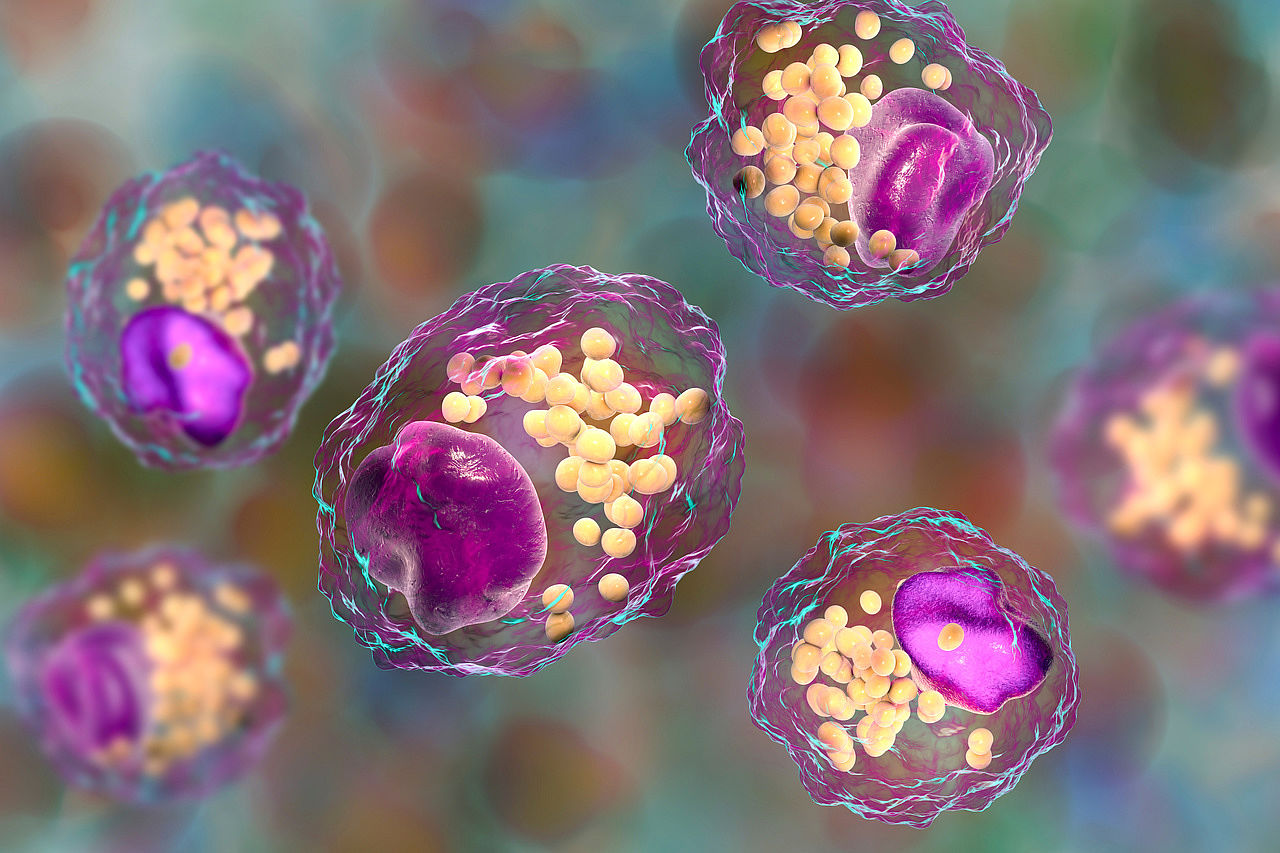
Macrophages are immune cells that remove cell debris from our body's tissues and perform other important functions. However, they also play a key role in the formation of atherosclerotic plaques in the walls of blood vessels. "Until now, we only knew how the macrophages are recruited to form atherosclerotic plaque and how they contribute to the development of the disease," says Dr Kami Pekayvaz, first author of the study. Certain messenger substances of the immune system - called CCL2 and MIF - play a decisive role.
In their study, the researchers used modern analytical methods to investigate in detail what happens after the recruitment of macrophages into the vessel wall. They deciphered how muscle cells in the vessel wall actively influence the local inflammatory environment - and made a surprising discovery: Vascular muscle cells secrete the messenger substances CCL2 and MIF, which are considered to be particularly harmful and have a strong effect on the function and distribution of macrophages.
However, this does not lead to an inflammatory influence on the phagocytes, but rather secures their normal, healthy functions. When the researchers prevented the release of CCL2 and MIF, the macrophages died or stopped working properly, "which accelerated the development of vascular inflammation and atherosclerosis," says Pekayvaz. In other words, depending on where they are in the vessel wall, the "bad guys" have a protective effect on the atherosclerotic process that kills millions of people worldwide. This discovery is very important for the future of cardiovascular research, because researchers around the world are working on anti-inflammatory therapies. "However, these therapies should not block the potentially beneficial effects of the messenger substances in certain areas of the vascular system and should therefore be cell-specific," says the Munich researcher, "in order to optimise the effects of these innovates treatment approaches".
Original publication: Mural cell-derived chemokines provide a protective niche to safeguard vascular macrophages and limit chronic inflammation, Pekayvaz et al., 2023, Immunity 56, 1-17, DOI: 10.1016./j.immuni.2023.08.002
Scientific contact: Dr. Kami Pekayvaz (kami.pekayvaz@med.uni-muenchen.de), Department of Internal Medicine I, LMU Munich
Source: Press release LMU Munich