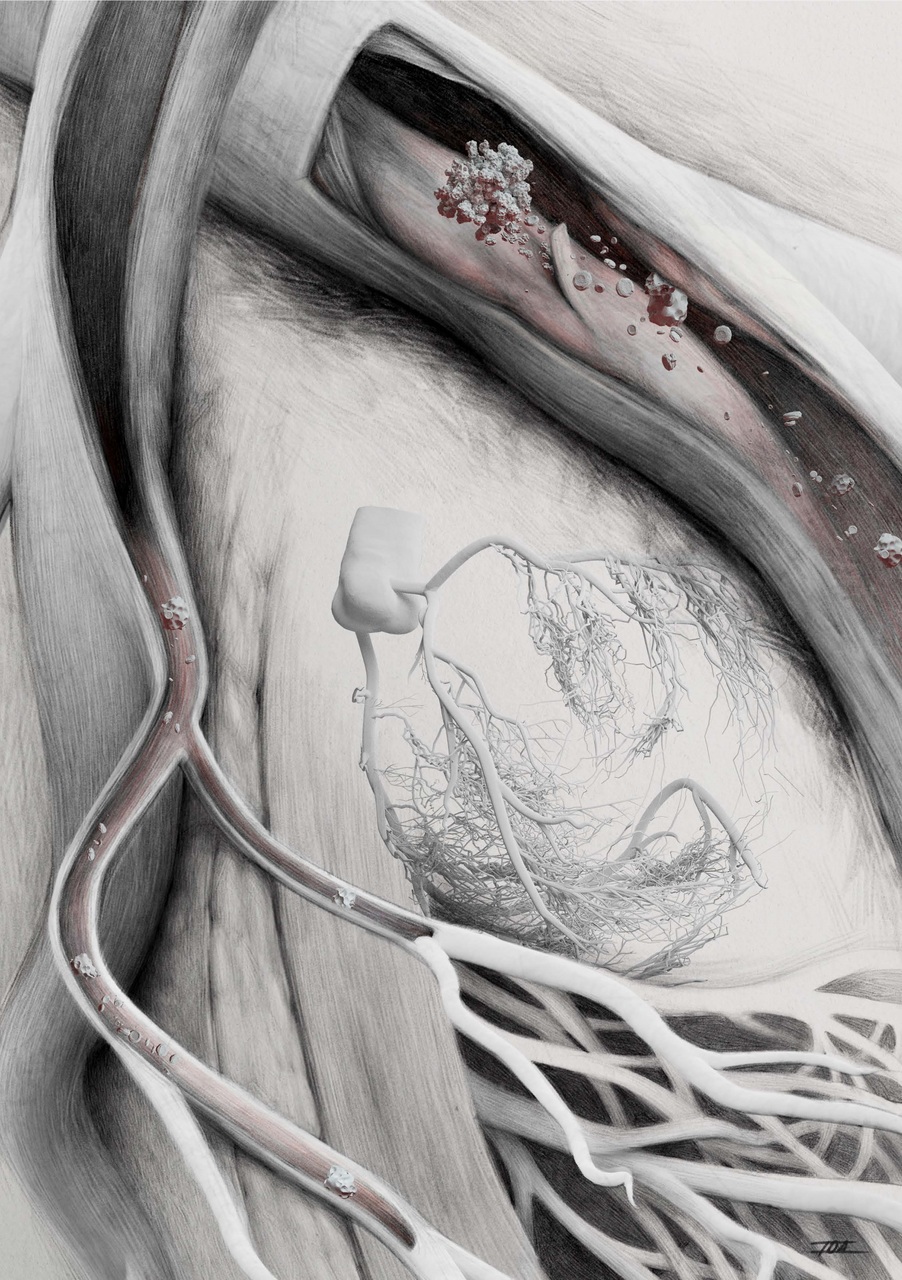
MINOCA is a specific form of heart attack: While the large coronary arteries are usually blocked or severely narrowed in an acute heart attack, this is not the case in MINOCA patients. The fact that no blood clot can be identified as the cause of the heart attack makes diagnosis and treatment of these patients, who account for about 15 percent of all heart attacks, more difficult.
To improve treatment options for patients with MINOCA, the Cardiac Surgery Research Group, in collaboration with colleagues from ETH Zurich and the University of Zurich, developed a clinically relevant pig model. Autologous microthrombi were used to simulate MINOCA in the coronary arteries. The studies showed that about 200 of these small blood clots are needed to reliably mimic the disease in the pig model. The comparability of the microthrombi used with the findings in MINOCA patients was demonstrated by electron microscopic analysis.
As is regularly observed in MINOCA, microthrombi in the pig model did not lead to stenosis, occlusion of the main coronary arteries or impaired myocardial perfusion. However, the ECG changes characteristic of the disease and an increase in cardiac biomarkers indicating oxygen deficiency and myocardial damage did occur. These findings were confirmed by cardiac magnetic resonance imaging, which showed multiple microdamages in the left ventricle. Histopathological examination of the heart tissue also showed that the blood clots were located in small arteries and arterioles and were associated with an inflammatory response. Thus, the pig model is consistent with the clinical features of MINOCA.
The new translational model may facilitate future in-depth studies of MINOCA and its underlying pathophysiology. On this basis, it will allow the development of new diagnostic and therapeutic approaches to benefit patients.
Dr Nikola Cesarovic, first author of the study, commented: "Our research provides a valuable tool to better investigate the underlying mechanisms of MINOCA. We hope that this model can help to address the diagnostic and therapeutic challenges associated with this disease and improve patient outcomes."
Prof. Maximilian Emmert, the last author of the study and head of the Cardiac Surgery Research Group, added: "It was fascinating to see how well and realistically the developed porcine model reproduced the MINOCA pathophysiology observed in our patients. As a next step, in addition to conducting long-term studies, we will now focus on investigating other pathologies underlying MINOCA disease and related pathologies in more detail."
Original publication: Development of a Translational Autologous Microthrombi-Induced MINOCA Pig Model. Cesarovic et al. Circulation Research. 2023;133:291–293
Scientific contact: Prof. Maximilian Emmert (maximilian.emmert(at)dhzc-charite.de), German Heart Center Berlin and Dr. Nikola Cesarovic (nikola.cesarovic(at)hest.ethz.ch), ETH Zürich
Contact: Pressemitteilung DHZC